
Given 0.62 g CuSO 4♵H 2O find the mass of water that would be driven off by heating

The following problems will help you to be able to do the math required for theįind the molar mass of the following hydrated and anhydrous salts. In the construction business this is known as Theīuilding materials will not rise above the 100☌ boiling point of water untilĪll of the water of hydration has been driven off. Hydrated building materials (such as concrete, gypsum wall board and plaster). Instead the water mixed with the concreteĬombines chemically with the materials in the cement and the resulting hydrates formĪ strong matrix that holds the concrete together and makes it strong.Īnother interesting example of the value of hydration is the incorporation of These chemicals absorb water by hydration. Of calcium silicates, calcium aluminate, calcium aluminoferrite and gypsum. The aggregate materials are the gravelĪnd sand that add strength to the final concrete. Concrete is made by mixing PortlandĬement with water and aggregate materials. WhenĬalculating the molar mass you add the molar mass of water (multiplied byĪn everyday example of hydration is concrete. One key point: the dot is not a multiplication sign. ForĬopper (II) chloride dihydrate and CuSO 4♵H 2O is copper (II) sulfate Hydrates are named using prefixes for the word hydrate (at right). A numerical coefficient gives the molar amount of water included in the Is used to separate the formula of the salt from the formula of the water of Hydrate in this reaction is called nickel (II) chloride hexahydrate.įormulas for hydrates are written using a dot convention: a dot

The so-called water of hydration of nickel (II) chloride ( NiCl 2) is six moles H 2O for every one mole of NiCl 2. Hydrated salts are characterized by the number of moles of water molecules per mole Hydrates but which have had all the water driven off, usually by heat. These salts, when they have absorbed water, are called The ions in some salts attract and form strong bonds with water Negatively charged parts within each molecule.
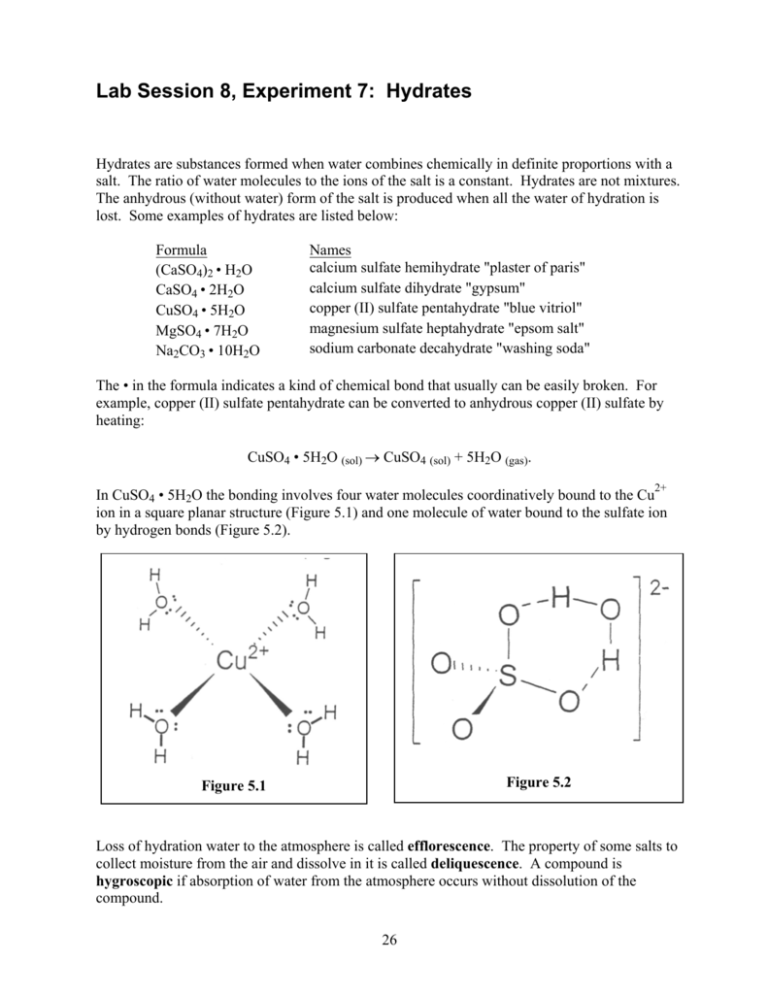
Water has a polar structure: it has positively and Some chemical compounds, especially inorganic salts, incorporate water into theirĬrystalline structures. Hydrated salt of known formula but unknown water content. Of known formula ( CuSO 4♵H 2O) and finding the formula of a The lab portion of this work conists in confirming the formula of a hydrate Nomenclature, and the molar mass of hydrates. Students willīe introduced to the concept of hydrated salts, anhydrous salts, hydrate This lab activity combines in-class problem-solving with lab work. The lab work has two objectives: first, confirm the formula of a hydrate with known formula and second, find the formula of a hydrate in which the salt formula is known but not the molar amount of water. Note for Teachers: Give out this lab handout at least one dayīefore doing this lab and assign the problems for homework.


 0 kommentar(er)
0 kommentar(er)
